
Fast-Track FDA Certification for Medical Devices: Key Tips for Success
October 22, 2025
Development of an Integrated Neuronal Excitability Recording System Combining Nerve Conduction Study (NCS) and Microneurography
October 26, 2025
Fast-Track FDA Certification for Medical Devices: Key Tips for Success
October 22, 2025
Development of an Integrated Neuronal Excitability Recording System Combining Nerve Conduction Study (NCS) and Microneurography
October 26, 2025Accelerating Innovation: End-to-End Engineering from Concept to FDA-Cleared Wireless Patient Monitoring
About Client
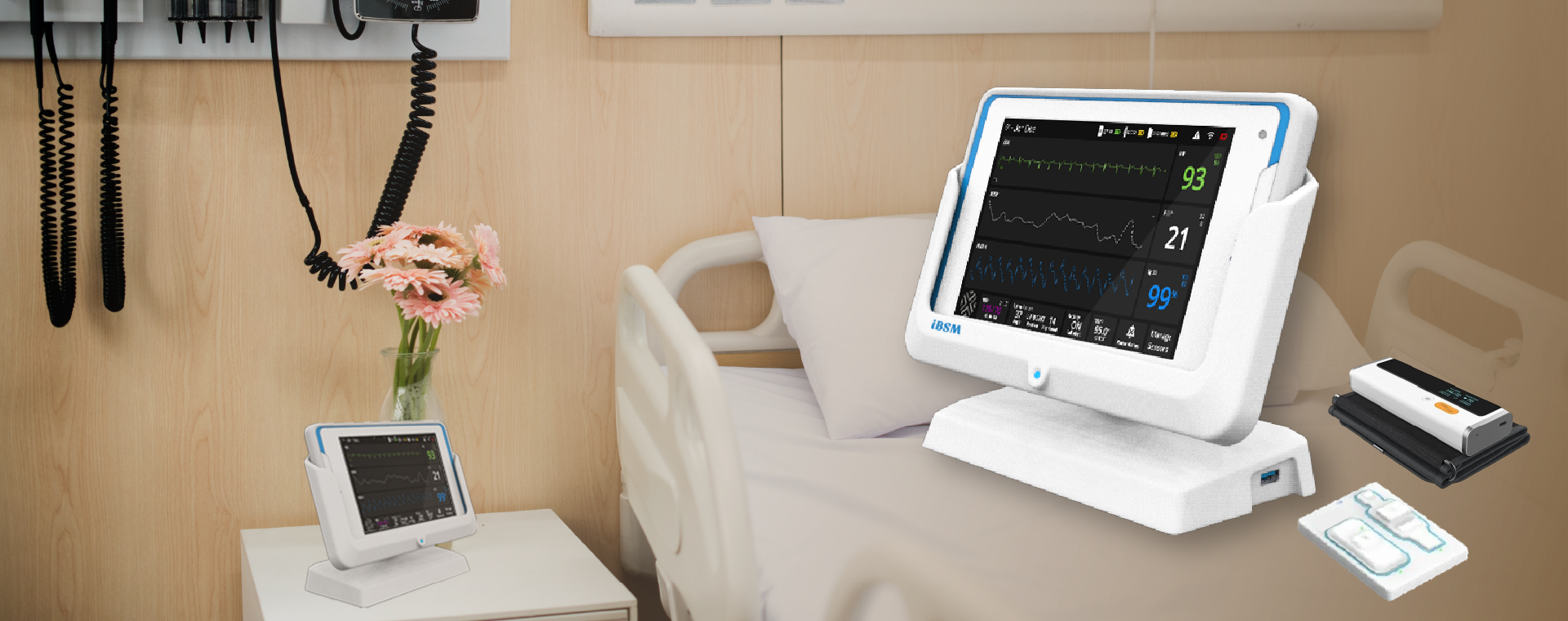
A global MNC leader in patient Monitoring products and entering wireless patient monitoring devices segment with a NPI/NPD product.
Client’s Challenge
- No product in the wearable medical device segment. Need to develop one for a growing market in the US and EU region.
- Novel medical device using wireless sensors, limited predicate devices
- Large number of physiological parameters to be monitored in real-time, multiple product codes
- Enhanced human factors engineering requirements, Single hand Docking mandatory
- Cloud connect data in a secure way FDA 510(k) clearance mandatory
iOrbit Solution
- End-to-end medical device product development of FDA Class II device: wireless patient monitoring system
- Medical grade app development along with a customized Docking Station for Hospital use
- Compliant Product DHF, Cybersecurity and Safety RM, Usability study, human factors validation study
- Comply with all certification standards and submissions
- Wireless coexistence and Cybersecurity tests
- FDA 510(k) submission (Ph-I)
- Design Implementation of the iBSM app
- Identification of a medical grade tab and enabling remote management of the same
- Mechanical design of the docking station and the associated hardware electronics
- Development and deployment of the associated cloud components to deliver the workflows
- Risk management for product safety and security
- Extensive Verification and Validation and generation of test reports
- DHF, IFU labelling and Medical Device File readiness for submission leveraging iOrbit's ISO 13485 processes
- Integrated testing of the product, including multi RF coexistence tests at TUV labs
- Human Factor Engineering, human factor validation tests and usability testing
- Design and development of product workflows and protocols
- Completion of all statutory IEC 60601 and ISO tests to meet FDA consensus standards
- 3rd party medical device cybersecurity testing and certification of all components
iOrbit Scope
- Leveraging expertise in digital healthcare technology, iOrbit designed the iBSM product platform to meet the requirements of a wireless patient monitoring system
- UX design and development of clinician-facing iBSM applications
- Seamless integration of wearables with bedside monitors
- Remote management of medical-grade tablets
- Mechanical and hardware design of an elegant and ergonomic docking station
- Cloud-connected medical device infrastructure design and workflow automation
- Safety, risk, and cybersecurity compliance
- Medical device verification and validation, usability, and human factors testing completed
- DHF, IFU, labeling, and documentation as per iOrbit's ISO 13485 process framework
- Third-party IEC/ISO testing and RF coexistence validation with TUV
Key Outcomes
- First-in-Segment Wearable Solution: Delivered a novel wearable patient monitoring device for US and EU markets, addressing a major portfolio gap.
- Regulatory Success: Achieved FDA 510(k) clearance (K243837) with full compliance to IEC/ISO, cybersecurity, usability, and RF coexistence standards.
- Seamless Clinical Integration: Enabled interoperability of wearable sensors with bedside monitors, clinician apps, and medical-grade tablets.
- Elegant & Practical Design: Developed an ergonomic single-hand docking station and robust hardware/software platform optimized for hospital workflows.
- Cloud-Connected Ecosystem: Designed secure cloud infrastructure and workflow automation for real-time physiological parameter monitoring.
- Patient Safety & Usability Validated: Completed rigorous Verification & Validation, Human Factors Engineering, and third-party cybersecurity testing.
- End-to-End Ownership: Delivered the complete DHF, IFU, labeling, and submission documentation under iOrbit's ISO 13485 framework.