Accelerating Innovation: End-to-End Engineering from Concept to FDA-Cleared Wireless Patient Monitoring
October 26, 2025
Revolutionized Neonatal and Elderly Care with Unobtrusive Fiber-Optic Monitoring
October 26, 2025
Accelerating Innovation: End-to-End Engineering from Concept to FDA-Cleared Wireless Patient Monitoring
October 26, 2025
Revolutionized Neonatal and Elderly Care with Unobtrusive Fiber-Optic Monitoring
October 26, 2025Development of an Integrated Neuronal Excitability Recording System Combining Nerve Conduction Study (NCS) and Microneurography

About Client
A leading hospital in the USA, part of a renowned healthcare system and affiliated with a prestigious medical university, recognized for excellence in patient care, research and innovation, with a dedicated Department of Neurology providing advanced therapies and modern technologies for comprehensive care of neurological disorders.
Client’s Challenge
- Bulky and complex – requiring multiple units and complicated connections.
- Difficult to operate – due to separate hardware modules and complex setup.
- Limited in capability – existing systems could not perform both large fiber or Nerve Conduction Study (NCS) and small fiber (microneurography) studies within a single integrated platform, which is only meant for NCS.
- Software dependency – reliant on the legacy QtracP and QtracS software, developed over 30 years of time and effort and supported only on Windows 7 and older versions.
- The client required a single, integrated solution capable of:
- Conducting both NCS and microneurography.
- Offering configurable stimulation and recording features.
- Replacing the legacy software protocols with a modern, reliable platform.
iOrbit Solution
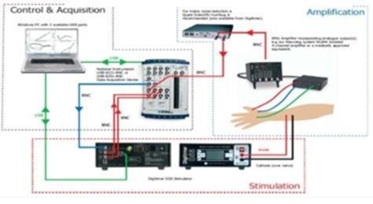
We developed NEERS (Neuronal Electrical Excitability Recording System) — a compact, fully integrated electrophysiology device combining stimulation, amplification, filtering, and data acquisition into one seamless platform.
This project was executed under our comprehensive
medical product engineering services framework, covering complete hardware and embedded system redesign.
Key Highlights
- Integrated Architecture: Replaced multiple Bostock components with one compact system.
- Dual Capability: Performs both large fiber (NCS) and small fiber (microneurography) studies.
- Dual-Processor Design: Enables high-speed acquisition, signal processing, and real-time protocol execution.
- Configurable Parameters: Adjustable current range, amplifier gain, and filter cut-offs.
- Modern Software Platform: Independently developed software replacing QtracP/QtracS systems.
- Validated Performance: Results closely matched gold-standard diagnostic systems.
For related neurology system development, explore our integrated neuromuscular diagnostic system (EMG & EIM) case study.
iOrbit Scope
The end-to-end medical device development scope included:
- Complete hardware redesign independent of the Bostock system
- Dual-processor embedded architecture
- High-gain, low-noise amplifier system development
- Implementation of advanced neurophysiological protocols into custom-built software.
- Clinical validation against legacy systems
medical device validation and verification processes to ensure performance accuracy and reliability.

Key Outcomes
- First-of-its-kind Prototype: Integrated nerve conduction study (NCS) and microneurography in a single device.
- Simplified Architecture: Eliminated multi-unit complexity.
- Modern Software Replacement: Removed dependency on outdated systems.
- Clinically Validated: Tested on hundreds of subjects with comparable results.
- Future-Ready Platform: Positioned for commercialization and regulatory progression.
For insights into regulatory acceleration strategies, read accelerating FDA clearance through smart regulatory planning.