
Independent Functional Testing of Patient Monitors from Multiple Manufacturers
October 27, 2025
Advancing Women’s Health: Innovative Home EMG/EEG Solution for Incontinence Treatment
October 27, 2025
Independent Functional Testing of Patient Monitors from Multiple Manufacturers
October 27, 2025
Advancing Women’s Health: Innovative Home EMG/EEG Solution for Incontinence Treatment
October 27, 2025Innovating Precision Care: Unified EMG and EIM Neuromuscular Diagnostic System
About Client
A leading medical technology company, working in collaboration with top hospitals and neurology departments across the USA and Europe. The client specializes in advanced diagnostic devices for neuromuscular and neurophysiological disorders, with a strong focus on precision, compliance and clinician usability.
Client’s Challenge
- Support simultaneous EMG and EIM measurements for comprehensive muscle assessment.
- Offer needle-based precision to ensure accurate signal acquisition during invasive diagnostics.
- Provide real-time visualization and clinician-friendly interfaces, streamlining clinical workflows.
- Meet IEC 60601 safety standards, align with ISO 13485 processes and be FDA submission-ready for regulatory approval.
iOrbit Solution
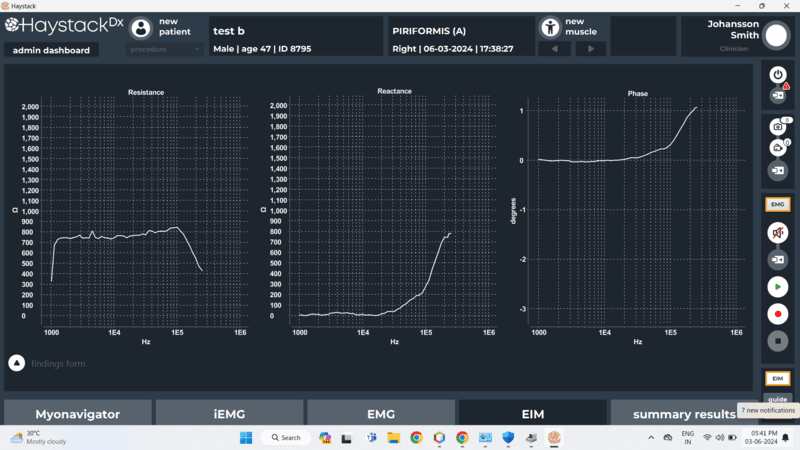
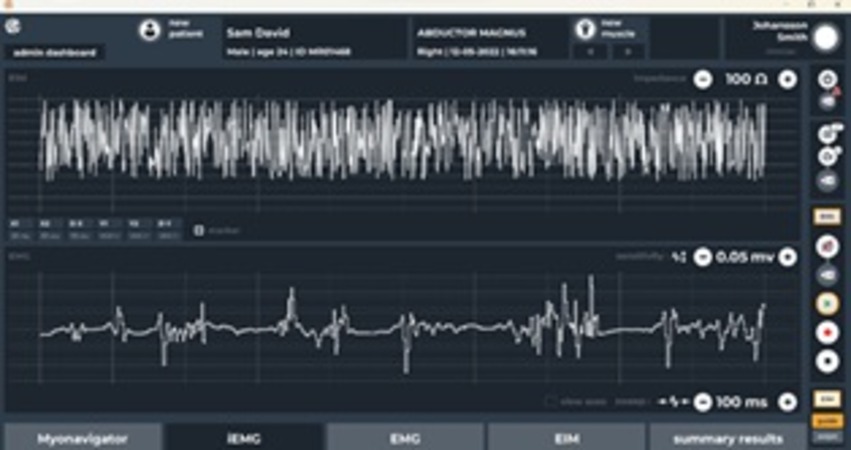
- Dual Measurement Capability – Supports both EMG and EIM, enabling comprehensive muscle health monitoring.
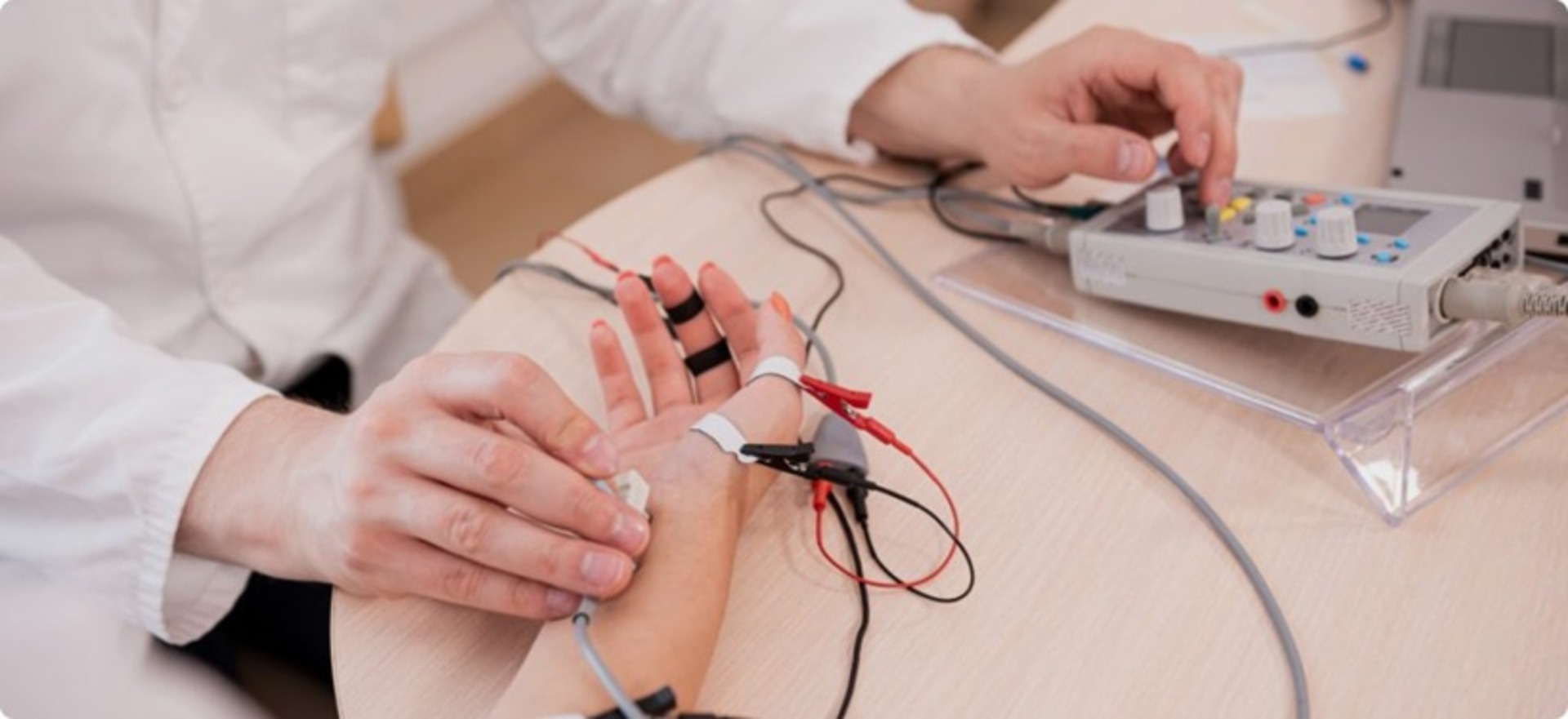
- Custom Needle – Invasive needle design with six metal contacts: two electrodes for EMG and four for impedance measurement, allowing simultaneous acquisition of multi-modal data.
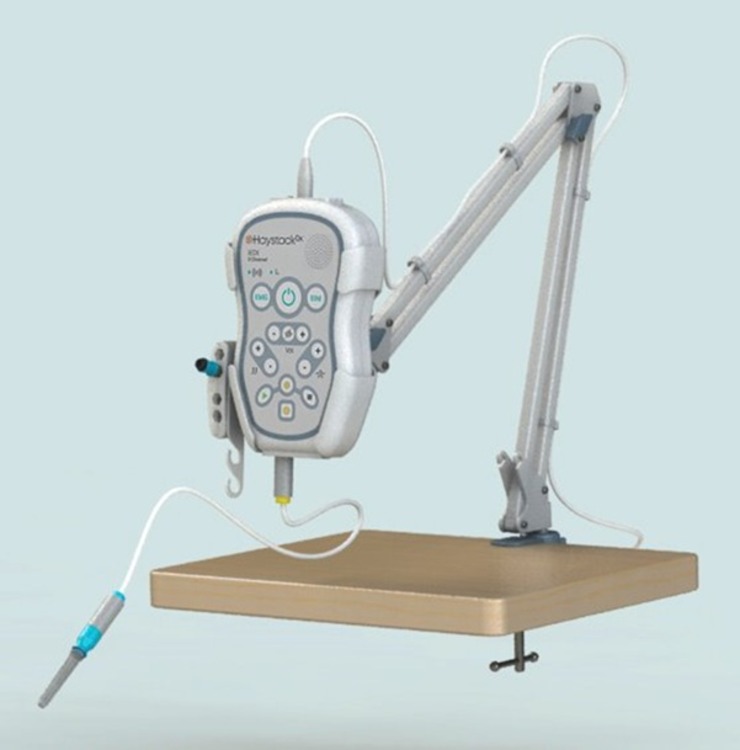
- Advanced Hardware Architecture – Built on a high-end processor and advanced technology platform for reliable control, stimulation generation and high-speed signal acquisition.
- Signal Conditioning Chain – Optimized with buffers, programmable-gain amplifiers and high-resolution ADCs to deliver accurate, noise-free EMG/EIM recordings.
- Safety & Compliance – Designed to meet IEC 60601 standards, aligned with ISO 13485 processes and FDA submission-ready for safe clinical use.
- Clinician Workflow Integration – Desktop software for clinicians to:
- Capture, visualize and record EMG and EIM signals in real time.
- Configure acquisition parameters.
- Manage patient data and generate automated reports.
iOrbit Scope
Key Outcomes
- First-of-its-kind integrated platform supporting both EMG and EIM in a single device.
- Improved clinical workflow by replacing fragmented tools with a compact, unified solution.
- Enhanced diagnostic precision with simultaneous EMG and impedance data capture using a needle-based approach.
- Real-time visualization and clinician-friendly data analysis tools with reporting capability.
- Designed, developed and documented per ISO13485 processes — paving the way for regulatory approvals and clinical adoption.
Conclusion
The EMG-EIM diagnostic system represents a breakthrough in neuromuscular assessment, combining EMG and EIM in one compact, safety-compliant platform. By integrating advanced AFE design, high-end processing technology and a clinician-focused software interface, the system delivers real-time, high-precision monitoring of muscle health. With its regulatory-ready design and deployment flexibility, the EMG-EIM platform is set to transform clinical practice in diagnosing and managing neuromuscular disorders.