
Powering Sustainability: Why Reusable Batteries Are the Future
October 22, 2025
Accelerating Innovation: End-to-End Engineering from Concept to FDA-Cleared Wireless Patient Monitoring
October 26, 2025
Powering Sustainability: Why Reusable Batteries Are the Future
October 22, 2025
Accelerating Innovation: End-to-End Engineering from Concept to FDA-Cleared Wireless Patient Monitoring
October 26, 2025
Fast-Track FDA Certification for Medical Devices: Key Tips for Success
To fast-track the FDA certification process for a medical device, especially under the 510(k) or De Novo pathway, a clear process flow diagram and strategic planning are critical.
FDA Fast-Track Certification Process Flow Diagram
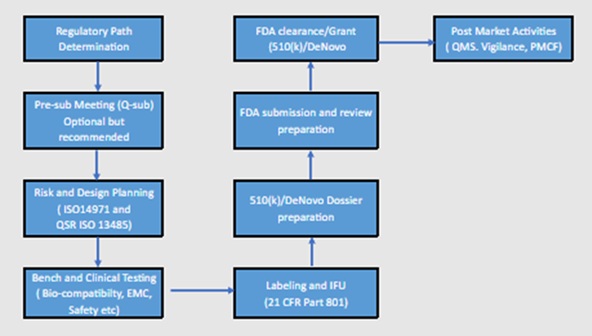
Tips to Fast-Track FDA Clearance with Effective Outcomes
1) Regulatory Strategy & Planning:
- Classify early: Use FDA product classification database to determine if 510(k), De Novo, or PMA is required.
- Use predicate mapping tools: For 510(k), compare it with well-documented devices with known clearance history.
- Use eSTAR format: Submit your 510(k) using FDA's structured eSTAR template for faster, fewer rejections.
2) Pre-Submission (Q-Sub)
- Schedule a Pre-Sub meeting (Q-Sub) to clarify specific topics and questions:
- Testing requirements
- Predicate acceptability
- Clinical study design (if required)
- Submit a Q-Sub at least 70 days before needing a feedback response date.
3) Design Controls & Testing
- Integrate design control documentation from the beginning: DHF, DMR, DHR (ISO 13485 / 21 CFR Part 820).
- Preplan:
- Biocompatibility (ISO 10993-1)
- Electrical Safety (IEC 60601-1)
- Software (IEC 62304)
- Cybersecurity (FDA Guidance)
4) Testing Strategy
- Get test reports through a FDA-recognized labs for BioComp, EMC, and electrical safety.
- Use FDA-recognized consensus standards and submit declarations of conformity.
5) 510(k) Submission Strategy
- Choose the right type:
- Traditional: Most common; 90-day review target.
- Special: For changes to an existing cleared device.
- Abbreviated: Uses recognized standards.
- Use eSTAR for faster document exchange.
- Prepare for interactive review and respond to additional info requests (AI letters)
6) Communicate Proactively
- Maintain open communication with the FDA lead reviewer once assigned.
- Respond to RTA (Refuse to Accept) or AI (Additional Information) requests quickly and completely.
Conclusion
Fast-tracking FDA certification requires more than just speed — it demands strategic foresight, technical excellence, and regulatory discipline. By aligning the product development with FDA expectations early on, leveraging structured tools like eSTAR, and maintaining proactive communication with the FDA, the chances of achieving first-cycle clearance significantly improve.
Furthermore, engaging experienced regulatory consultants, using FDA-recognized standards and test labs, and adopting a robust documentation and QMS framework ensure that the submission not only proceeds quickly but withstands regulatory scrutiny with confidence.
In conclusion, a well-planned, standards-aligned, and FDA-engaged approach is the key to accelerating the medical device’s journey to market — ensuring faster access for patients and competitive advantage for the business.