
Bringing Real-Time Medical Device Data into Hospital Management System (HMS)
January 14, 2026
Transforming Care Delivery in Eldercare Homes with a Human-Centered Connected Care Solution
January 22, 2026
Bringing Real-Time Medical Device Data into Hospital Management System (HMS)
January 14, 2026
Transforming Care Delivery in Eldercare Homes with a Human-Centered Connected Care Solution
January 22, 2026From Concept to Care: End-to-End Product Engineering of a CPAP/BiPAP Respiratory Device

About Client
A major Medical Device manufacturer in Asia introducing indigenous Sleep Apnea Respiratory devices with full design and manufacturing ownership thereby replacing the earlier OEM white labelled distributed device.
Client’s Challenge
To have a complete ownership and control of product specs and design, post transfer from iOrbit, of a new customized cPAP/BiPAP product, for full scale manufacturing and commercial release. Performance to be better than the current OEM white labelled product.
- Limited in-house resources bandwidth to design and develop a completely new cPAP/BiPAP machine
- Complex design around air flow dynamics and accurate control
- Multi mode operation within the system
- Enhanced usability requirements
- Highly ergonomic and aesthetic Industrial Design (ID)
- Full fledge Design control DHF documentation, ready for regulatory submissions
iOrbit Approach / Solution
- End to end product development of CE Class IIa device
- Full Electro-Mechanical design and resolution of prior other products issues around noise control
- Medical grade App. development as an accessory for clinical settings
- Compliant Product DHF, Cybersecurity and Safety RM, Usability study, HF validation study
- Comply with all certification standards and submissions
- Design Implementation of the iBSM app
- Risk management for product safety.
- Extensive Verification and Validation and generation of test reports
- DHF, IFU labelling and Medical Device File readiness for submission leveraging the iOrbit 13485 processes
- Integrated testing of the product.
- Design and development of product workflows and protocols
- Completion of all statutory IEC/ISO tests to meet FDA consensus standards.
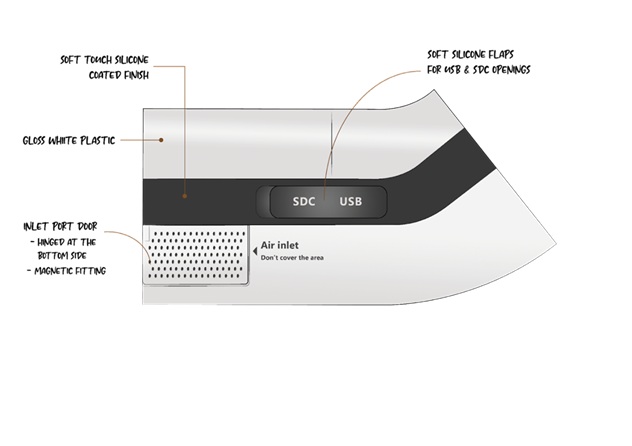

Key Outcomes
- Leveraging the expertise in state-of-the-art Respiratory products, iOrbit designed the cPAP/APAP/BiPAP product to meet the requirements of PRS and URS
- UX design and development of clinician-facing cPAP applications
- Seamless integration of cPAP OTS standard components
- Mechanical and hardware design of an elegant and ergonomic station
- Safety, risk compliance
- Verification & Validation, usability, and human factors testing completed
- DHF, IFU, labeling, and documentation as per iOrbit’s ISO 13485 process framework